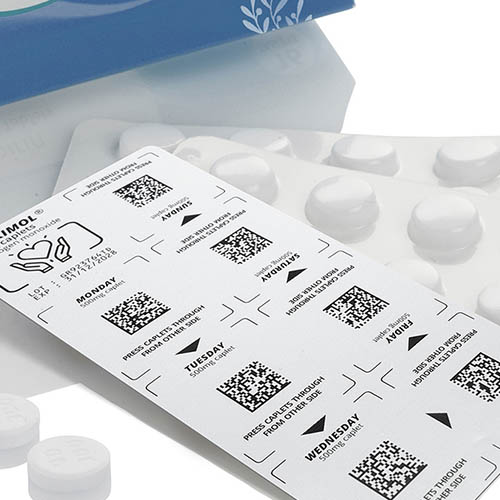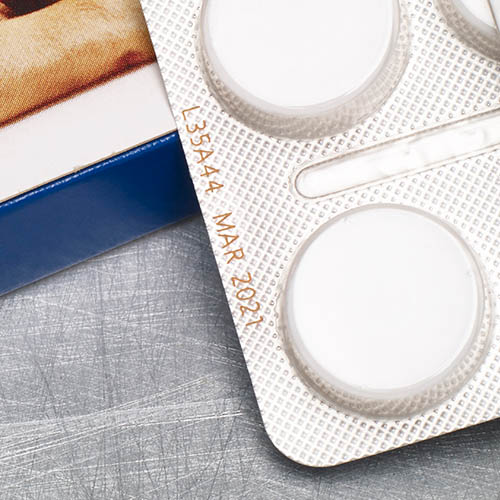
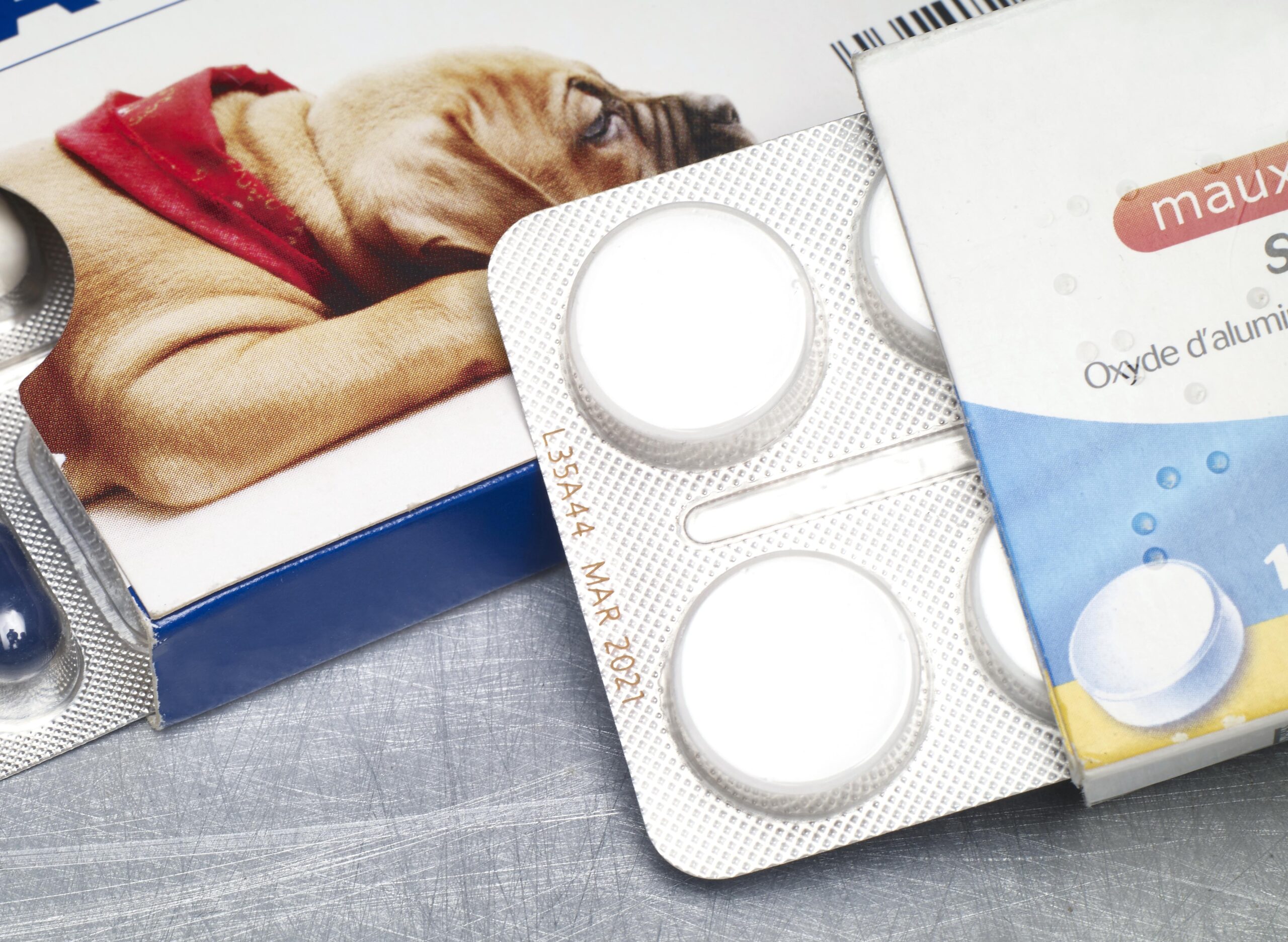
Labeling blister packs
Precise coding on blister foils - legible, tamper-proof and production-safe
Blister packs are an integral part of pharmaceutical and medical technology. To ensure traceability and product safety, they must be reliably labeled. Clear, durable print images for batch numbers, best-before dates and serialization data are particularly important here. Depending on the material and production speed, different technologies are used that can be seamlessly integrated into existing lines.
Get in touch with usWhy is the labeling of blister packs important?
Traceability and product safety
In the pharmaceutical and medical technology industries, the complete traceability of medicines and medical products is required by law. The labeling on blister packs makes it possible to trace each unit back to the production batch.
Counterfeit protection on the market
Counterfeit medicines are a problem worldwide. Individual serial numbers and machine-readable codes on the blister packs ensure that each pack is clearly identifiable.
Optimize logistics and warehousing
Clearly legible labelling on blister packs facilitates allocation in warehousing processes and ensures an efficient flow of goods - both during packaging and transport.
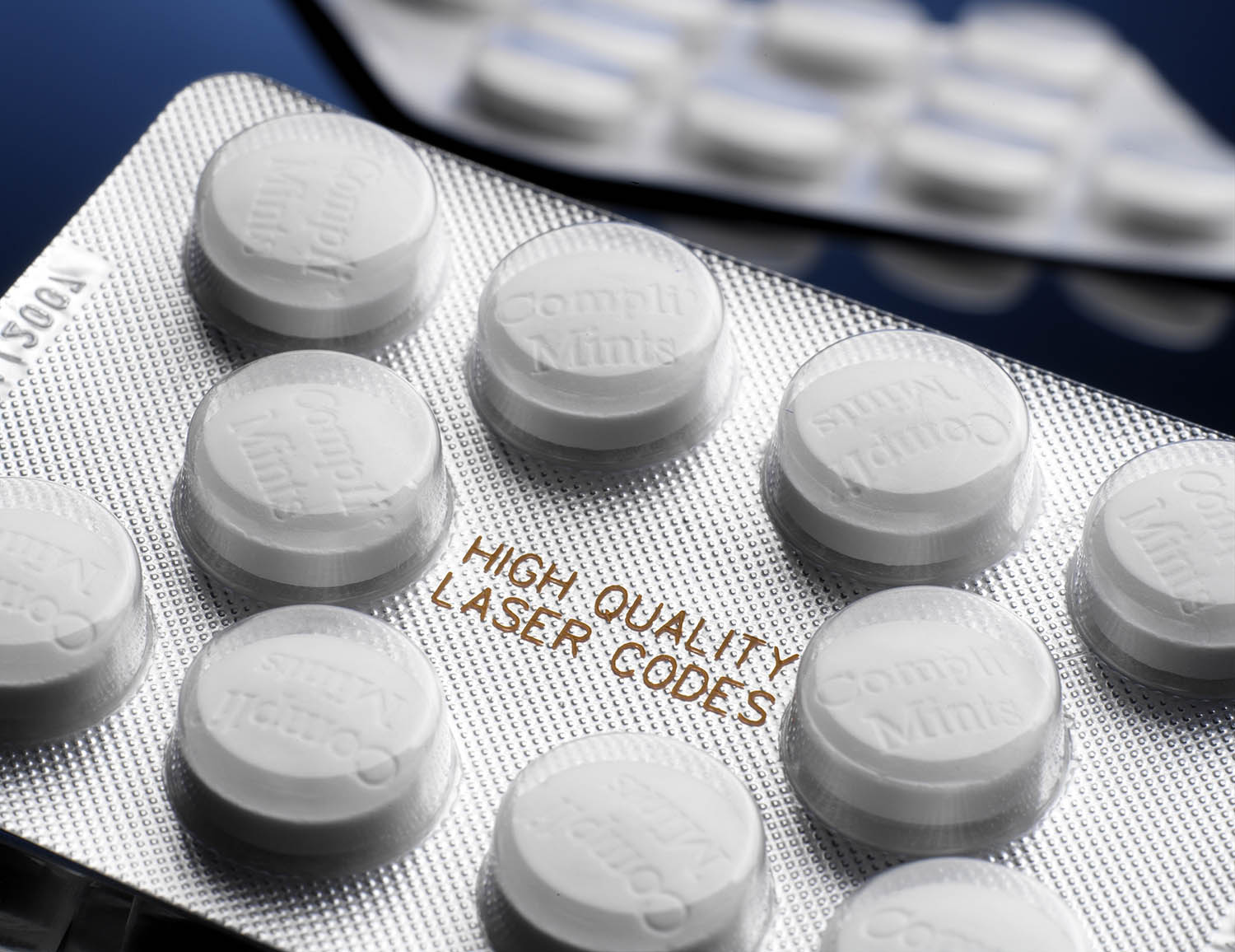
Challenges in the labeling of blister packs
Blister packaging places special demands on printing technology, adhesion and legibility. The material properties and high production speeds in particular require precisely coordinated systems.
High production speeds
Pharmaceutical products are often produced in cycle times of several hundred packages per minute. The marking must run synchronously without impairing the production flow.
Variety of materials and surface finishes
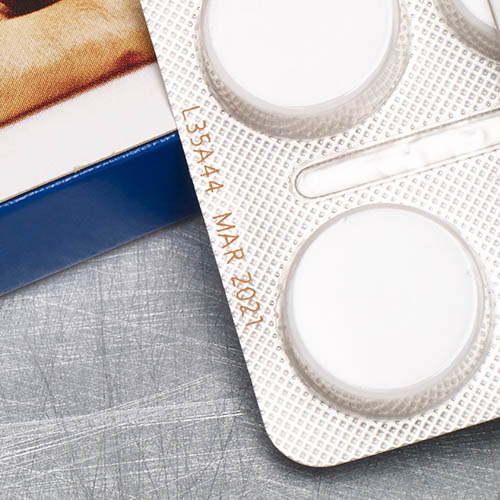
Blister films are often made of aluminum, PVC or polypropylene and are often pre-printed. The printing technology must be adapted to the respective materials in order to ensure clear and abrasion-resistant marking.
Counterfeit protection and serialization
In the pharmaceutical environment in particular, it is important that serial numbers and codes are applied to the packaging so that they cannot be changed. The marking must be tamper-proof and machine-readable in order to meet international standards.
Suitable technologies
Various printing technologies are used for marking eggs and egg cartons. Gentle treatment of the sensitive shell, food-safe inks and reliable adhesion to carton packaging are crucial. The following technologies have proven themselves in practice:
Get in touch with us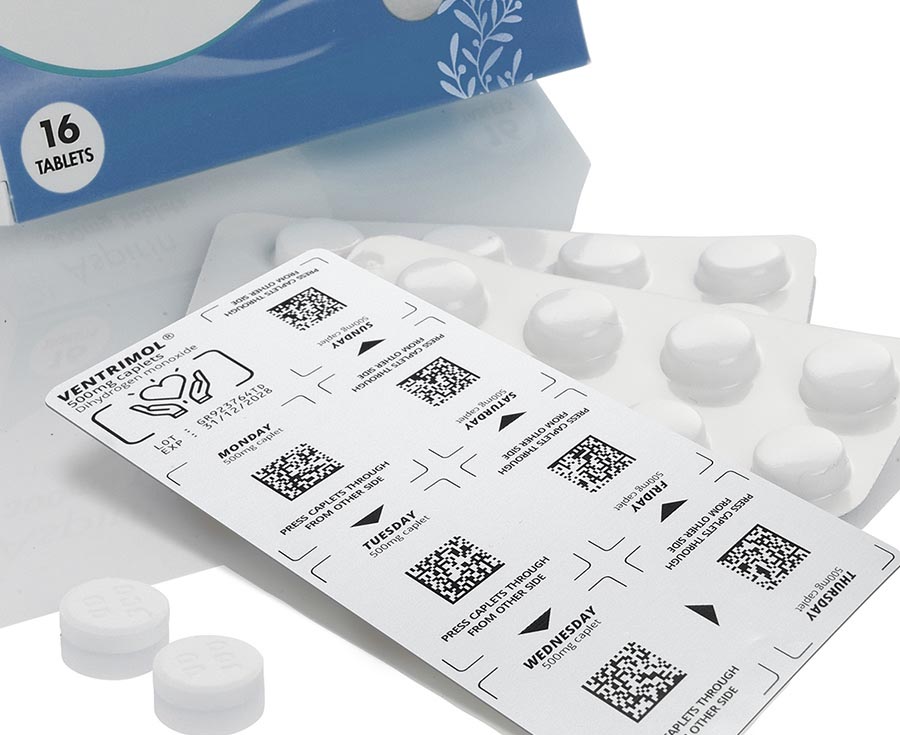

Laser marking
Laser marking offers a permanent and high-quality marking solution for blister packaging. Particularly suitable for aluminum foils and certain plastics. The marking is done without inks or solvents, which reduces maintenance.
- Permanent engraving without consumables
- High print quality for complex codes and graphics
- Ideal for aluminum blisters and plastic-coated materials

Thermal transfer printing (TTO)
Thermal transfer printing is ideal for flexible blister packaging such as films. The technology enables the printing of variable data, best-before dates and batch numbers. The labeling is usually done on pre-printed films as a supplement to the already applied layouts.
- High-resolution printing for variable data
- Ideal for flexible film packaging
- Addition of pre-printed information
Thermal Inkjet (TIJ)
TIJ systems enable high-resolution marking directly on blister packs or secondary packaging. They are often used to apply production data, QR codes or serial numbers to pre-printed blister foils.
- Precise printing for QR codes and text information
- Contactless labeling with high resolution
- Supplementary printing of pre-printed films

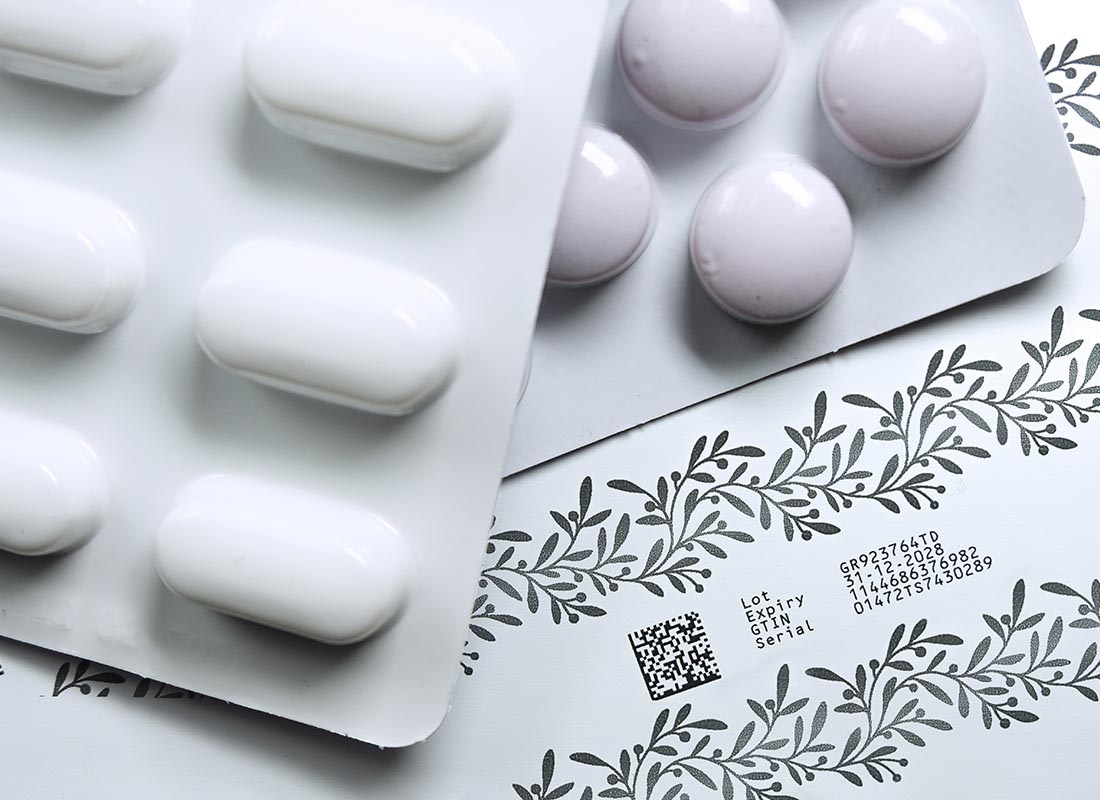
Special solution: Domino K600G
The K600G is a specialized digital printing solution that makes it possible to print directly onto the blister chambers themselves - at the single-dose level. In contrast to other technologies, no pre-printed foil is required here. Printing takes place flexibly during the production process so that each chamber can be serialized individually.
- Serialization of each individual chamber (single dose)
- High resolution of 600 dpi for clear, machine-readable codes
- No pre-printing necessary - fully variable during the running process
- Perfect for pharmaceutical applications with strict traceability requirements
The CHROMOS way to the optimum solution
Requirements analysis
We work with you to examine the specific requirements of your production line - from line speed to the material properties of the blister packs.
Technology selection and adaptation
Based on the analysis, we select the right printing technology and adapt it precisely to your production conditions. We attach great importance to smooth integration into existing processes.
Test printing and process integration
We carry out test prints under real conditions and check the legibility, adhesion and durability of the marking. We then integrate the printing systems seamlessly into your existing line.
Support and optimization
Our team accompanies the commissioning and offers you comprehensive technical support. We are also on hand to assist you with changes to your line - flexibly and reliably.
Our tip: Get advice from our experts
Blister packs require precise and reliable marking - whether on the film, the packaging or directly on the chamber. An individual analysis of the requirements ensures that production processes run efficiently and in compliance with regulations.
Get in touch with usApplication examples
Contact us
Our experts will be happy to help you. Get in touch with us!